SurgiBind® Tendon Protect designed for preserving motion in surgical tendon management procedures without substantial tissue loss
Kerecis, the company pioneering the use of fish-tissue in cellular therapy and tissue regeneration, will debut its latest innovation, SurgiBind® Tendon Protect, at the American Podiatric Medical Association’s (APMA) Annual Scientific Meeting in Grapevine, Texas, July 24-27, 2025. The new biologic graft is designed for tendon procedures where no significant tissue loss is present and will be spotlighted during educational and interactive sessions throughout the conference.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20250723324805/en/
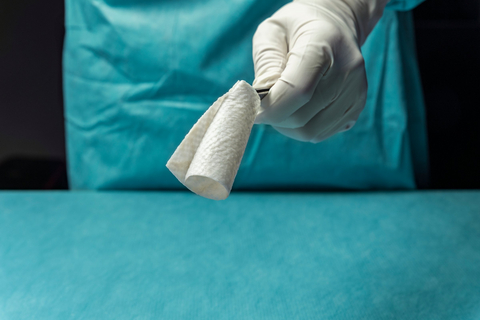
SurgiBind® Tendon Protect by Kerecis
APMA 2025
As part of its presence at APMA 2025, Kerecis will exhibit at Booth #805 and host a clinical education event, “Taste of Iceland,” on Friday, July 25, from 7:00 to 9:00 PM at Mission Plaza, Gaylord Texan Resort. During the session, Dr. James Cottom, DPM, will present “Gliding into Enhanced Tendon Outcomes with Intact Fish Skin,” a talk exploring the clinical use of SurgiBind® Tendon Protect in tendon repair procedures. Attendees will also have the opportunity to learn more about the broader application of intact fish-tissue grafts while sampling traditional Icelandic cuisine.
Designed for Tendon Protection and Surgical Efficiency
Each year, an estimated 500,000 tendon procedures are performed in the United States, many of which aim to preserve or restore movement following tendon injury. SurgiBind® Tendon Protect is intended to be wrapped around a repaired tendon during surgery and is cleared for implantation to reinforce soft tissue where weakness exists.
The product is a solid, intact fish-tissue based graft, sustainably sourced from North Atlantic cod, and is bioresorbable, non-crosslinked, and designed to be sutured and positioned with ease in the surgical field.
“Tendon Protect was developed in response to a need consistently identified by surgeons. Namely, coverage for repaired tendons that is straightforward to use and compatible with surgical workflows,” said Fertram Sigurjonsson, Founder and CEO of Kerecis. “The result is a graft that is structurally sound and easy to handle, offering an alternative to mammalian-derived products.”
Clinical Use
SurgiBind® Tendon Protect is intended for use in ambulatory surgical centers (ASCs) and hospital outpatient departments (HOPDs), providing a physical covering around repaired tendons where there is no significant tissue loss. Its strength and pliability support secure placement and suture fixation and reduces the risk of readmissions or revision procedures related to scarring or adhesion, factors that can increase overall treatment costs and affect reimbursement.
Audit-Ready™
To support providers, Kerecis offers coding and reimbursement guidance through its Audit-Ready™ program, designed to align with U.S. payor requirements, reduce administrative burden, and streamline claims and documentation.
Available Nationwide
SurgiBind® Tendon Protect marks the continued expansion of the Kerecis SurgiBind® portfolio, which now includes options for general surgical, trauma, and tendon-related procedures. The product is currently available across the United States through Kerecis’ direct sales force.
About Kerecis
Kerecis, founded by Fertram Sigurjonsson, develops intact fish tissue derived products for cellular therapy, tissue regeneration, and protection. When grafted onto damaged human tissue or implanted, the patented material supports the body’s own processes to heal and regenerate. Because no disease-transfer risk exists between cold-water fish and humans, Kerecis products are only gently processed and retain their similarity to human tissue. The gentle processing preserves the material’s original three-dimensional structure, maintaining its inherent natural strength, complexity, and molecules (such as fatty acids). Clinical studies show that Kerecis products heal wounds faster than competitors. Kerecis is the only global manufacturer of medical devices containing intact fish-tissue and is the fastest growing company in the U.S. xenograft biologics skin market. Products include SurgiBind®/SurgiClose®, GraftGuide®, MariGen®, and Shield™ for various medical applications. Committed to the UN Sustainable Development Goals, Kerecis uses sustainably sourced Icelandic fish processed with renewable energy. Kerecis is a part of Coloplast, a leading supplier of intimate healthcare products. For more information about Kerecis and its clinical research, visit www.kerecis.com.
Trademarks and registered trademarks are the property of their respective owners.
View source version on businesswire.com: https://www.businesswire.com/news/home/20250723324805/en/
“SurgiBind® Tendon Protect was developed in response to a need consistently identified by surgeons. Namely, coverage for repaired tendons that is straightforward to use and compatible with surgical workflows,” said Fertram Sigurjonsson, Founder and CEO of
Contacts
Media Relations Agency
(952) 697 5220




