As an expert in providing solutions to assist virology and microbiology research, Creative Diagnostics recently announced the availability of its comprehensive Antibody-dependent Enhancement (ADE) assay services, including the evaluation of FcR-mediated ADE, C-ADE, and iADE. This critical tool will help researchers understand the mechanism of ADE and accelerate the development of vaccines and therapeutic monoclonal antibodies.
Antibody-dependent enhancement (ADE) refers primarily to the in vivo effect of pathogen infection. In 1964, Hawkes and his team at the Australian National University first formulated the hypothesis of "antibody-dependent enhancement of infection" of arboviruses and named it ADE. ADE suggests that viral replication is facilitated, rather than inhibited in low concentrations of immune serum. A number of viruses have been found to exhibit the ADE effect, including Ebola virus, West Nile virus, dengue virus, hepatitis C virus, and rabies virus.
The exact mechanism of the ADE effect is still unclear, but it is generally believed that the increase in viral infectivity is mainly through the Fc receptor pathway, which promotes the uptake of viral antibody complexes by target cells. Understanding the mechanism of ADE, identifying the antigenic determinants associated with ADE in viruses, and modifying them will contribute to the development of safer and more effective vaccines. In addition, since ADE has been shown to depend on both antibody concentration and neutralization properties, it is important to assess the effect of antibody responses induced by specific immunization regimens on ADE.
ADE assays and their in vitro experiments can serve as benchmarks for careful evaluation of new generation vaccines or targeted drugs. More importantly, ADE effects are an essential part of the body's immune regulation, and a clear understanding of their mechanisms can provide opportunities and directions for research and development of new drugs for infectious diseases with well-defined pathogen infections.
Creative Diagnostics offers comprehensive ADE assay services for the evaluation of FcR-mediated ADE, C-ADE and iADE. The company helps clients understand the mechanisms of ADE, clarify the conditions that lead to ADE and identify the factors that control activation. The ADE services can identify antigenic epitopes of virus-induced ADE, make recommendations for avoiding these epitopes in vaccine design, discover potent virus-neutralizing epitopes and other non-neutralizing but protective epitopes, and guide the design of future protective vaccines and therapeutic monoclonal antibodies. Creative Diagnostics is committed to recommending safe and effective adjuvants to prevent ADE.
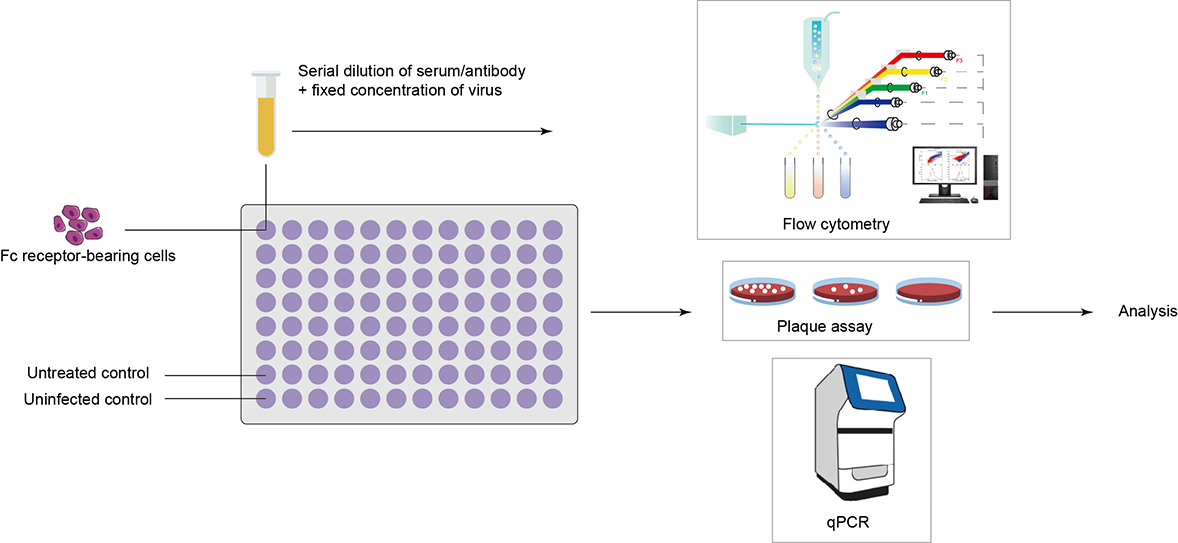
Creative Diagnostics has established stable cell lines expressing FcγRI, FcγRII, FcγRIII to evaluate the effectiveness of research test articles. The team employs a rigorous approach that incorporates flow cytometry, plaque assay, or qPCR depending on the client's needs. Internal controls consist of known concentrations of purified enhancing and isotype antibodies, while each plate includes untreated and uninfected controls to minimize variability. To optimize efficiency and allow parallel testing or increased replicates, Creative Diagnostics has standardized its analyses using 96-well plates, reducing the amount of test materials required.
For more information on how this assay can benefit your research, please visit https://antiviral.creative-diagnostics.com/antibody-dependent-enhancement-ade-assay.html.
About Creative Diagnostics
Headquartered in New York, Creative Diagnostics is a consulting and experimental service provider specializing in virology and microbiology. The company provides comprehensive solutions to conquer obstacles in virology and microbiology research, from high-security infrastructure provision, biosafety regulation elucidation, to expert viral system assistance.
Media Contact
Company Name: Creative Diagnostics
Contact Person: Thomas Schmitt
Email: Send Email
State: New York
Country: United States
Website: https://antiviral.creative-diagnostics.com/